Introduction
Since the outbreak of the COVID-19 pandemic began in 2020, it continues to pose an unprecedented global challenge in healthcare. Even though several vaccines have been approved, targeted therapies are still required as vaccines do not provide complete protection. Additionally, they have issues related to safety, storage, and efficacy against mutant strains.
In the early stage of the COVID-19 pandemic, researchers opted for repurposing of drugs approved for other indications or those which exhibited a positive benefit/risk balance in other conditions. This approach, however, failed since none of the repurposed drug molecules showed positive outcomes. Antiviral drug candidates such as Remdesivir or Bamlanivimab/Etesevimab combination, although approved, are clinically restricted in improving the clinical management of mild to moderate patients. Moreover, they do not provide a complete cure.
Even though there has been limited progress, there are expectations for the treatment of COVID-19 infected patients. The therapeutic strategy that is successful in elucidating a positive outcome would likely be a game-changer and would also become the preferred choice, particularly amongst the elderly.
In this case study, we explore and summarise the emerging patent landscape with a focus on therapeutic molecules that are under development for the treatment of COVID-19 infections.
Methods
For the patent landscape and to identify molecules under development, we performed the patent search queries using our own LucidSearch search engine and the SureChemBL database. LucidSearch was also used to retrieve COVID-19 related articles and validate the search keywords.
In SureChemBL, the query field was used for performing a simple freestyle text search using the Lucene syntax – THERAPY AND COVID related keywords COVID19 OR COVID-19 OR Corona virus OR Coronavirus.
Results
Time-series analysis
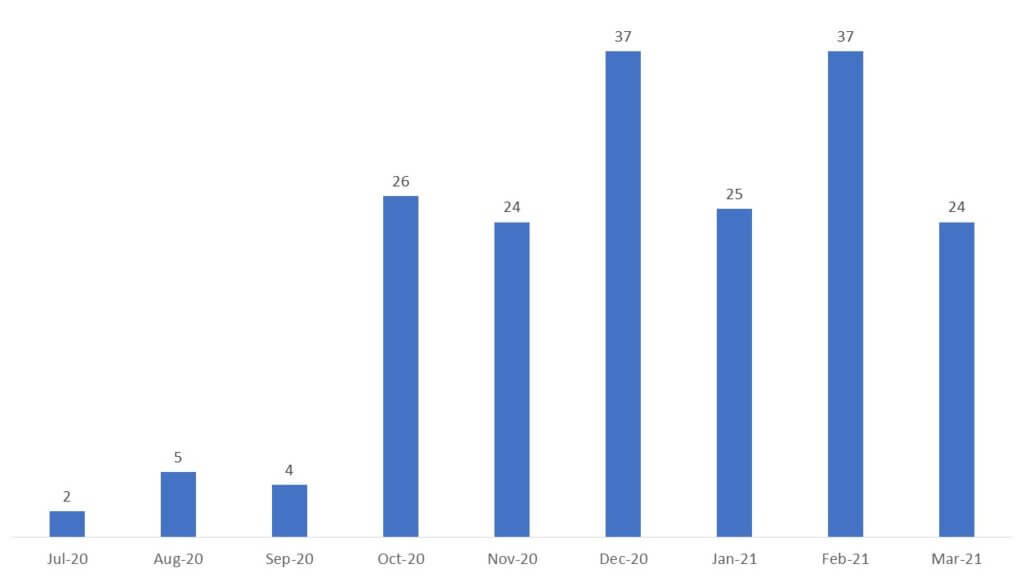
As of March 25, 2021, 184 patent families with patents published between July 2020 and March 2021 were retrieved. The time-series analysis indicates that the number of patent publications is closely related to the outbreak of the pandemic with the first patent published in July 2020. Until today, 86 patents have been published in 2021 and the number is expected to grow further (Figure 1).
The trends indicate high interest and desire from the scientific community both within academic institutions as well as industrial organizations in identifying new approaches to halt progression and transmission of the disease.
Figure 1. Time-series analysis of COVID-19 therapeutic patents.
Key therapeutic modalities
The analysis indicates an interest in antibodies, peptides, nucleic acid-based therapy, and novel synthetic molecules (Table 1)
- Key therapeutic strategies involve targeting complex molecular interactions involved in coronavirus infections, replication or suppressing cell death through NAMPT, RIPK1, RSK or BTK inhibition
- Immune-mediated strategies include targeting immune dysregulation or modulation of host responses through regulatory T–cells, CD39, CD154 or elucidating Th1/Th2 responses
- Other methods that can prove to be an attractive option due to their rational design include oligonucleotides, novel synthetic molecules targeting the viral structure like thiazolidine, TUDCA or using NO formulations that can be inhaled.
Table 1. Top organisations and modalities.
|
Company/Institution |
Modality |
|
Aqualung Therapeutics |
Anti-NAMPT antibodies |
| AJK Pharmaceuticals |
Antimicrobial peptides |
|
Atea Pharmaceuticals |
Purine nucleotide phosphoramidate |
|
Atriva Therapeutics |
RSK inhibitors |
|
Cadila Pharmaceuticals |
Sulfoximine derivatives |
|
Christian-Albrecht University of Kiel |
RIPK1 inhibitors |
| Gilead Sciences |
2-imino-5-oxo-imidazolidine inhibitors |
| Junior University |
Regulatory T–cells |
| Massachusetts Institute of Technology |
Circular strained zymogen |
| MC Sciences |
Etifoxine |
| Antisense oligonucleotides | |
|
Purinomia Biotech |
Anti-CD39 antibodies |
|
BTK kinase inhibitors |
|
|
Lactoferrin |
|
| Tarsus Pharmaceuticals |
Isoxazoline parasiticides |
|
University of California |
Agent enhancing Th1 response and/or reduces Th2 response |
|
TREM1 inhibitors |
|
|
The University of Nebraska |
Thiazolide derivative |
|
Thirty holdings limited |
Nitrite formulation |
|
TFF2 polypeptides |
|
|
CD154 antibodies |
|
| TYME Inc |
Tauroursodeoxycholic acid (TUDCA) |
| Hong Kong Baptist University |
Patentiflorin A analogs |
| University of Antwerp |
Mucin isoforms |
Summary and Perspectives
- The patent information provides strong intellectual support of the ongoing R&D in the identification of therapeutic agents for the treatment of COVID-19. Despite being in the early stage of development, they may be beneficial in the long-term control of COVID-19
- Even though multiple strategies are being explored, the use of combination therapy could be more potent in reducing risk, viral load and disease progression.
- There are limited attention and knowledge gaps in the treatment of mutant strains, posing a bigger challenge in therapeutic management. It is believed that patients infected with emerging and novel mutations of COVID-19 may require different treatment strategies.
#COVID19 #pandemic #patents #Remdesivir #Bamlanivimab #Etesevimab #LucidSearch #SureChembl
Sources
https://www.nature.com/articles/s41418-020-00720-9
https://www.nytimes.com/2020/10/15/health/coronavirus-remdesivir-who.html
https://www.fda.gov/media/145801/download