Gene therapies can be the answer to treating, preventing, or curing various diseases. The most dominant areas for gene therapy applications are oncology, nervous system diseases, cardiovascular, ophthalmology and blood disorders.
Over the past few years, clinical trials and gene therapies that made it to the market attracted attention to the two main categories of gene delivery systems: viral and non-viral.
A previous Lucidquest article covered their characteristics and shared the results of the research the Lucidquest team conducted on clinicaltrials.gov. database regarding their use per therapeutic area through the years. This article will provide an overview of forecasts, impactful advancements, and our perspective on the future.
Gene Therapy Market and Vector Market Reports reveal emerging trends — non-viral vectors on the rise.
The emergence of non-viral vector therapies raises concerns that viral vector gene therapies, specifically AAVs, will be under threat.
- According to a report published by Allied Market Research, the gene therapy market was valued at $6 billion in 2021 and is expected to reach 46.5 billion by 2030 with a growing CAGR of 22.8%.
- As per the Globe Newswire report, AAV’s Global Downstream Processing market is projected to grow from USD 27.4 billion in 2022 to USD 48.1 billion by 2028 at a CAGR of 15.3% from 2023 to 2028″.
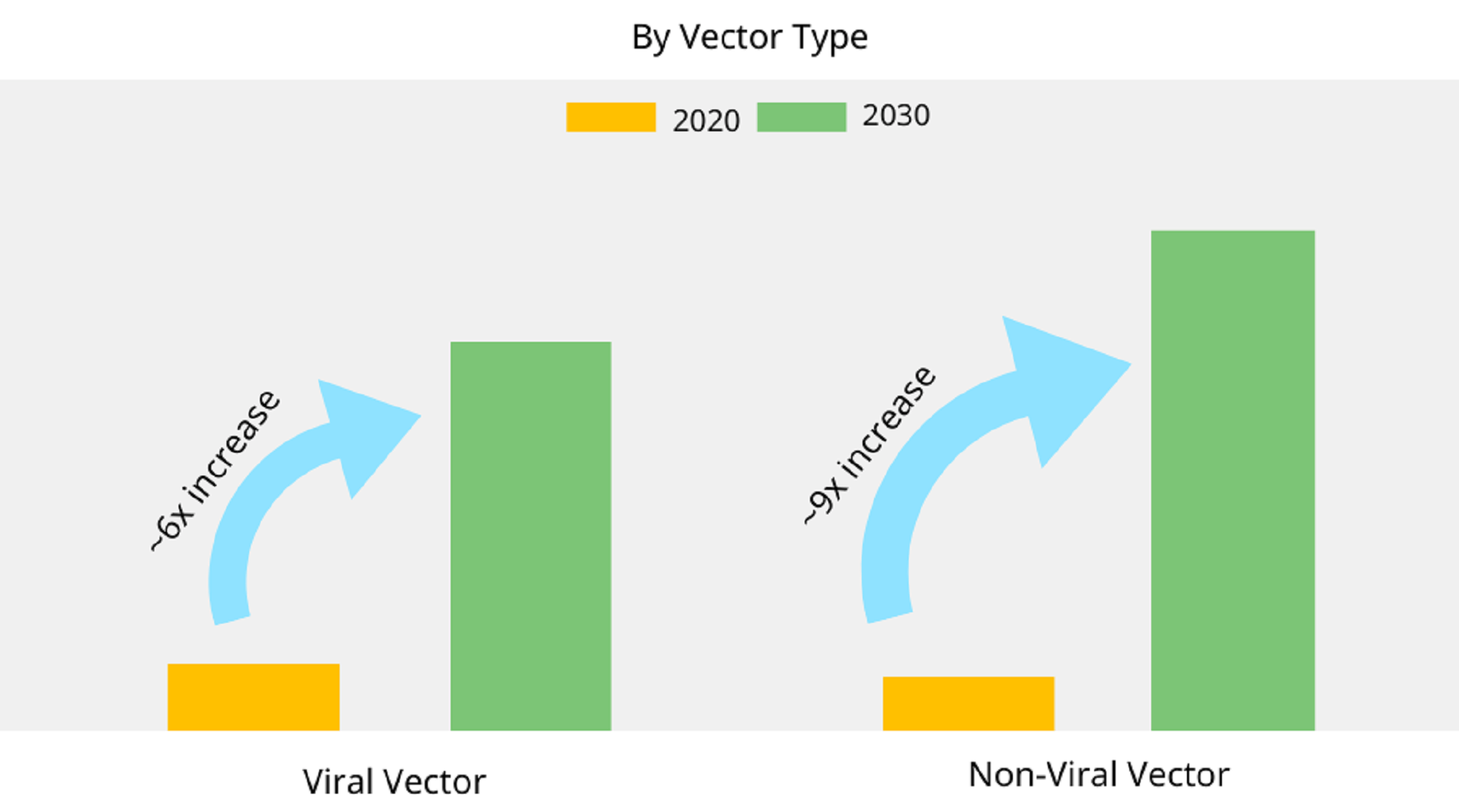
- A Markets and Markets report mentions that in 2021, non-viral vectors held the largest market share in North America compared to viral ones. Also, as shown in Image 1 (adapted by Allied Market Research), non-viral vectors are expected to maintain market dominance by 2030.
- Regarding non-viral vectors, a report by Insights Partners stated that the liposomes market was worth USD 3.6 million in 2019 and is projected to reach approximately USD 7 million by 2027, at a CAGR of 8.8%.
- The Nanomaterials Market Report by Grand View Research makes a revenue forecast for USD 32.76 billion in 2030 at a CAGR of 14.8% from 2023 to 2030.
AAVs: Will their benefits outweigh immunogenicity and manufacturing challenges?
To determine if AAVs are under threat as gene therapy approaches, we sought to review the benefits, the challenges, and the pharma advancements for each delivery system for different therapeutic areas.
AAVs: Αn attractive and widely used Gene Transfer System
AAV vectors constitute an attractive and widely used gene transfer system, with many clinical trials proving their usefulness and several products based on them already available in the market.
AAVs have the following significant advantages: the ability to transduce non-dividing cells, low pathogenicity, long-term and stable transgene expression, and the ability to be produced at high titers.
AAV Drugs are already in the market
AAV-based drugs like Zolgensma (Novartis) for treating spinal muscular atrophy and Luxturna (Spark Therapeutics/Novartis) for Leber congenital amaurosis are already available.
AAV Challenges: Immunogenicity and high manufacturing cost
AAV therapies come with two significant limitations: immunogenicity and high manufacturing cost.
When administered to a patient, AAVs can activate the complement pathway due to their capsid proteins, leading to immune responses and inflammation. Furthermore, AAVs may trigger the production of neutralizing antibodies, especially if the patient has been exposed to the wild-type virus before. Additionally AAVs have broad tropism, thus rendering cell/tissue specific targeting challenging. Finally, the fact that AAV preparations may contain empty capsids, makes calculation of accurate dosing difficult.
Efforts to eliminate the above limitations complicate the manufacturing process (including limitations in transfection methods) and lead to higher manufacturing costs. Currently, AAV therapies are expensive, with treatments such as Luxturna costing $425,000 for each affected eye.
Are Non-viral Gene Therapies the answer we were looking for?
Non-viral vector gene transfer systems is an umbrella term that refers to vectors such as plasmids, nanoparticles, liposomes, polymers, and their combinations. Non-viral transfer systems have become increasingly popular due to their numerous benefits. The technology used to develop mRNA vaccines during the Covid-19 pandemic was based on non-viral vector gene transfer systems.
Non-Viral Vectors promise low immunogenicity and low production costs.
Non-viral vectors have several advantages over viral vectors:
- They have lower immunogenicity compared to non-viral vectors.
- They are easier and less expensive to manufacture.
- They have a high packaging capacity and low mutagenicity.
Drugs based on non-viral vectors are available in the market.
The availability of drugs based on non-viral vectors in the market goes beyond Covid-19 vaccines.
There is Onpattro (Alnylam Pharmaceuticals) for the treatment of polyneuropathy in people with hATTR amyloidosis and Doxil/Caelyx (Janssen) for the treatment of breast and ovarian cancer (Doxil/Caelyx in combination with bortezomib is used for the treatment of multiple myeloma).
Moreover, Spinraza (Biogen) is used for treating spinal muscular atrophy, and Collategene (AnGes) for treating critical limb ischemia.
Non-viral vectors are subject to efficacy challenges
The primary concern with non-viral vectors is their efficacy. Gene transfer efficiency, specificity, and transgene’s long-term and stable expression need optimization. For this reason, until now, non-viral vectors had been exclusively used in clinical practice. However, this may change in the future.
Moreover, non-viral vectors do not come with zero side effects. They have lower toxicity than viral vectors but still exhibit some toxicity due to the materials used for their generation (e.g. gold nanoparticles). And though they are less immunogenic, they activate immune responses that must be tackled.
Ophthalmology, Nervous System Diseases, and Vaccines are leading areas for Gene Therapy Applications.
The expected growth of the gene therapies’ market size comes from innovations and investments in non-viral-based and viral-based drugs.
We already see important AAVs and non-viral vector innovations and advancements in:
- ophthalmology
- nervous system diseases
- vaccines
Ophthalmology: Luxturna: The first FDA-approved gene therapy for a genetic disease
Despite the complications (among which are macular holes, surgical complications, and hypotonia), we see innovative advancements and active trials for both viral and non-viral vectors in ocular gene therapy. The most important milestone is the Luxturna FDA approval (2017) for treating Leber congenital amaurosis.
AAVs in Ophthalmology
Luxturna is an AAV2-based vector that encodes RPE65 and has been proven safe and effective. AAVs show greater transduction efficiency when transducing RPE photoreceptor cells after subretinal administration compared to other types of viral vectors like adenoviruses.
Also, VEGF therapies for treating wet AMD with drugs such as Aflibercept (fusion protein), ranibizumab, and bevacizumab have been developed.
Though these drugs are effective, the need for repetitive intravitreal injections and the associated adverse effects explain why clinical trial results were more promising than the real-world data.
Some clinical trials to watch in this field are:
- the trial for RGX-314 (Regenxbio), an AAV8 vector encoding a soluble anti-VEGF m-AB fragment
- the trial for ADVM-022 (Adverum), an AAV vector carrying a coding sequence for Aflibercept
Non-Viral Vectors in Ophthalmology. DNA Nanoparticles (NP) stands out.
DNA Nanoparticles (NP) (Copernicus Therapeutics), a single plasmid DNA compacted with (PEG)-substituted 30-mer lysine peptide (CK30PEG), which enters the nucleus via the nucleolin-dependent endocytic process stands out in the non-viral vectors field. NP outperformed AAVs in transducing the retina and RPE after intravitreal injection in non-human primates.
Other milestones in the field of non-viral vector therapies in ophthalmology are:
- Eyevensys’s electrotransfection system
- Intergalactic Therapeutics’ IG-002 targeting ABCA4 retinopathies Link
- ProQR Therapeutics’ sepofarsen for LCA10 patients.
Regarding sepofarsen, in spite of the fact that the results of the phase 2/3 trial did not meet the primary endpoint, EMA suggested that ProQR runs a second 2/3 phase clinical trial before submitting an MAA — a recommendation showing the RNA therapies potential in treating LCA10.
However, with challenges in finding more LCA10 patients, along with the associated costs of running another trial, ProQR decided not to proceed and to allocate its resources to the Axiomer RNA-editing program. Meanwhile, it focused on finding a partner to further develop its retinal antisense oligonucleotide (AON) programs for LCA10, USH2A, and PR-RHO.
Gene Therapies in Neurology: Challenging but promising
The inherent complexity of the nervous system and the challenge of passing the blood-brain barrier explain why developing gene therapies for neurological diseases is demanding. However, CRISPR technology and the development of virus-like particles (VLPs) have substantially elevated research and fostered innovation in the field.
Viral vectors: TSHA-102 for Rett syndrome and TSHA-120 for giant axonal neuropathy.
TSHA-102 for treating Rett syndrome and TSHA-120 for giant axonal neuropathy deliver promising results. Astellas, in October 2022, entered into an agreement with Taysha Gene Therapies to strategically “invest a total of $50 million to acquire 15% of the common stock of Taysha and to receive an exclusive option” to license TSHA-102 and TSHA-120.
Also, there are more clinical trials using AAVs and promising positive results for treating:
- Parkinson’s Disease (Clinical trials NCT00985517, NCT04167540, NCT00195143 and NCT01973543)
- Altzheimer’s Disease (Clinical trials: NCT00087789, NCT00876863, and NCT03634007)
- Huntington’s Disease (Clinical trials: NCT05243017)
- Frontotemporal dementia (Clinical trial: NCT04747431)
Non-viral gene therapy for neurological diseases is still in the preclinical stage.
Polymer-based, lipid-based, and nanoparticle-based vectors, primarily focusing on Parkinson’s and Alzheimer’s treatments, are being used, with most of the trials being at the preclinical stage.
Non-viral vectors and AAVs in vaccines
There are AAV-based vector vaccines and non-viral system vaccines (such as mRNA) on the market that can prevent infectious diseases or cancer. Numerous clinical trials are currently underway to ensure strong responses with minimal adverse effects.
AAV-based vector vaccines are widely used due to their advantages in the vaccines’ space.
AAV-based vector vaccines were used to prevent infectious diseases since they can induce strong and lasting antibody responses that, in some cases, outperform the responses induced by other vaccine types such as VLPs or recombinant proteins. However, their limited transgene capacity and pre-existing immunity are still challenges calling for a solution.
On the other hand, non-viral systems like mRNA vaccines have certain advantages, as they
- do not integrate into the genome
- stimulate B and T cell immune response
- are non-infectious
However, there are drawbacks primarily related to their stability and immunogenicity.
Covid-19 mRNA vaccines: most prevailing among all Covid-19 vaccines
GlobalData reports that the covid-19 vaccines market had a value of $13.6 billion in 2021 and is projected to reach $19.5 billion by 2026. Currently, mRNA vaccines are the most prevalent in the market. Recent advancements, such as the launch of a nanoparticle vaccine by Novavax and a recombinant protein vaccine by Sanofi and GSK, will not reshape the market, as Pfizer and Moderna vaccines will maintain their throne.
Cancer vaccines market is expected to grow. Clinical trials going on.
From the first cancer vaccine in 1976 for bladder cancer to the first FDA-approved cancer vaccine — Provenge for prostate cancer — and the use of VLPs, there have been tremendous knowledge and innovation advancements.
According to a report from Allied Market Research, the market for cancer vaccines was worth $4,188 million in 2019 and is projected to grow to $7,303 million by 2027, with a compound annual growth rate of 12.6%. The most commonly used technology has been recombinant cancer vaccines, while viral-based vaccines have been used the least.
Ongoing clinical trials for cancer vaccines of various types and delivery vehicles are, among others:
- NCT03164772 for non-small cell lung carcinoma (Ludwig Institute for Cancer Research and collaborators: Cancer Research Institute (CRI), Boehringer Ingelheim, MedImmune LLC, CureVac, PharmaJet, Inc)
- NCT00204607 for malignant melanoma (University Hospital Tuebingen)
- NCT04382898 for prostate cancer (BioNTech SE)
- NCT03313778 for solid tumors (ModernaTX, Inc. and collaborator: Merck Sharp & Dohme LLC)
Besides Provenge, cancer vaccines (of various types) available in the market are:
- Abecma for multiple myeloma
- HEPLISAV-B for hepatitis B
- Cervarix, Gardasil, and Gardasil-9 for HPV
The future of AAV vectors. Will an upcoming AAV divestment wave reshape the market?
AAVs have been the top viral system and a leading cell and gene therapy innovation. However, there is a shift, and the non-viral vectors market share will grow significantly, raising concerns about AAVs being pushed to the sidelines in the future.
Forecasts estimate the AAVs market will grow at a CAGR higher than 10% by 2035. According to the available data, over 550 AAV therapies are currently being assessed in various stages of development. Additionally, over 80 industry stakeholders manufacture AAVs, and over 30 players are involved in AAVs platform development.
Meanwhile, there are many ongoing clinical trials for AAVs, and partnership activities in the AAVs field from 2017 to 2021 increased at a CAGR of almost 50%. Last but not least, over 50 AAVs-related start-ups have emerged over the previous ten years.
On the other hand, certain companies have decided to disinvest from AAVs. Mark Gergen, CEO of Poseida Therapeutics, during a podcast in the summer of 2022, explained “how and why the company is moving away from AAV delivery in favor of nanoparticle delivery” Also, Takeda recently decided to stop discovery and preclinical research with AAVs and prioritize focusing on other programs like TYK2 inhibitor TAK-279 for the treatment of autoimmune diseases.
Considering market data, the pros and cons of each delivery system, and the therapeutic areas involved, answering if non-viral vectors will threaten AAVs is quite complex. Some companies are shifting away from AAVs while others still invest in them.
As long as companies focus on improving viral-based and non-viral-based technologies, monitoring how effectively manufacturing, cost, efficiency, toxicity, and immunogenicity challenges will be addressed is essential. Collecting and analyzing data from many clinical trials and real world use will show how successfully each of the two main delivery systems can solve unmet needs and dominate the market.
Do you want to stay updated with the latest trends and advancements in viral and non-viral markets?
Or do you need a partner to support you with competitive intelligence through the new drug development process?
Contact us and gain best-in-class market insights. Learn how we help you make informed decisions.
#genetherapy, #AAV, #viralvectors, #polymers, #nanoparticles, #Luxturna, #Spinraza #clinicaltrials, #retinaldiseases, #CNS, #cancervaccine
Sources:
Global Adeno-associated Virus Vectors (AAVs) Downstream Processing Market Size
Gene Therapy Market Revenue Trends and Growth Drivers | MarketsandMarkets
Gene Therapy Market Size & Growth | Analysis By 2030
Liposome Drug Delivery Market Trends | Global Forecast to 2027
Nanomaterials Market Size, Share & Growth [2023 Report]
Overcoming the Challenges for AAV Gene Therapies – PharmaFeatures
Nanoparticles in the clinic: An update – PMC
Emerging patent landscape for non-viral vectors used for gene therapy – PMC
Non-viral Vectors in Gene Therapy: Recent Development, Challenges, and Prospects – PMC
https://clinicaltrials.gov/ct2/show/NCT03066258
https://clinicaltrials.gov/ct2/show/NCT03748784
Ocular gene therapies in clinical practice: viral vectors and non-viral alternatives – ScienceDirect
Summary of the Eighth Annual Retinal Cell & Gene Therapy Innovation Summit 2023
The use of viral vectors in vaccine development
Advances in mRNA Vaccines for Infectious Diseases
Non-viral Delivery Systems of mRNA Vaccines for Cancer Gene Therapy
Cancer Vaccines – Cancer Research Institute (CRI)
Moving On from AAV with Poseida Therapeutics CEO Mark Gergen
Layoffs loom as Takeda trims early-stage efforts in AAV gene therapy, rare hematology
Image Credits: