Gene therapy is a medical approach that treats a disease by correcting the underlying genetic cause. It is applied by introducing a new gene into the defective cell (either wild type gene or another that reverses/ameliorates the pathological phenotype).
The main obstacles to safe and efficient gene therapy are related to suboptimal gene transfer and the need for long-term and stable, tissue-specific gene expression. All these issues can be “controlled” to some extent by an appropriate design of gene delivery systems. The two main categories of gene delivery systems are viral and non-viral systems, and we will review them in this article. More specifically we will present the finding after analyzing gene delivery systems used in clinical trials as listed on Clinicaltrials.gov.
Global manufacturing vectors market size. Forecasts and key players.
The need for more efficient and safer gene therapy and the growing financing opportunities have led to the rapid evolution of several new vectors (viral and non-viral). Both report forecasts and analyst predictions trends show growth.
According to a Bisresearch report published in 2021, “the global manufacturing vectors’ market was valued at $1.5 billion in 2020 and is expected to reach $27.03 billion by 2031”.
The agreement between AstraZeneca and Oxford Biomedica for the development of an adenovirus-based Covid 19 vaccine and the expansion of Thermo Fisher Scientific manufacturing capabilities serve as proof of the rapidly growing vector manufacturing market.
A Research and Markets report published in December 2021 identified
Boehringer Ingelheim, Catalent, Inc., FUJIFILM Holdings Corporation, Merck KGaA Inc., Oxford Biomedica plc, Takara Bio Inc., Thermo Fisher Scientific Inc., as the key players in the space, while PharmTech also mentions Astellas Gene Therapies, Bayer, Bluebird Bio, Intellia Therapeutics, Regenxbio, Sangamo Therapeutics, Vertex Pharmaceuticals, and Voyager Therapeutics.
Gene Delivery Systems recapped. Pros and Cons.
The two main categories of gene delivery systems are viral and non-viral systems. Viral gene transfer systems include adeno-associated virus (AAV), adenovirus, lentivirus, retrovirus, herpesvirus, and poxvirus. Non-viral systems include naked DNA, plasmid, liposomes, polymers, transposons, and nanoparticles.
Viral transfer systems
Adenoviruses belong to the family Adenoviridae, have no envelope, and their genetic material is dsDNA.
Adenoviruses combine high transduction efficiency with low pathogenicity, but the fact that they are highly immunogenic leads to toxicity.
In addition, the viral genome is not integrated into the host genome, resulting in transient low-level transgene expression.
Adeno-associated viruses belong to the Parvoviridae family. They lack an envelope, and their genetic material is ssDNA which mostly acts episomally and does not integrate into the host cell genome.
Adeno-associated viruses are commonly used to transduce cells with a slow dividing potential, such as cardiomyocytes.
Retroviruses have RNA genetic material, which is incorporated into the host genome as linear dsDNA after reverse transcription.
The most well-characterized and widely used retrovirus is the murine leukemia virus (MLV).
The main disadvantage of this transfer system is the phenomenon of insertional mutagenesis due to the random integration of the viral genetic material into the genome of the target cell, as well as the fact that it can only transduce dividing cells and not quiescent cells.
It allows continuous expression of the transgene, and this is the reason why it is often used at the clinical level.
Herpes simplex virus’s (HSV) most significant advantages are its large capacity for transgene insertion (40-150kb) and its high tropism towards neurons.
Its genome does not integrate into the host cell genome, is highly immunogenic, and can induce a strong antitumor immune response.
Lentiviruses have ssRNA genetic material of the Retroviridae family. The most commonly used lentiviral vectors are those derived from HIV (human immunodeficiency virus) and SIV (simian immunodeficiency virus).
They are often used in gene therapy because they offer long-term transgene expression, even in non-dividing cells.
In an effort to increase the safety of gene therapy, different lentiviral vectors have been engineered through genomic editing, and now third-generation lentiviral vectors with specific characteristics are being used.
Non-viral transfer systems
Plasmids are the simplest form of vector for gene delivery. They comprise dsDNA, and we can insert DNA fragments or genes into them.
They are usually administered with liposomes or a gene gun, are safe with low immunogenicity, and are mainly used for vaccines. Their main problem is short-term transgene expression.
Liposomes are macro or nano-size particles surrounded by lipid bilayers. Gene delivery efficiency of liposomes varies depending on their size, composition, loading efficiency, charge, and stability.
The most frequently used liposomes are cationic and smart. They can uptake larger DNA constructs than viral vectors, they protect the cargo, and they have sustained drug release.
Nevertheless, among their disadvantages are low stability and low half-life.
Nanoparticles have a small size and high surface area/volume ratio, constituting an efficient and promising rapidly evolving non-viral system.
For nanoparticle generation, several organic or inorganic materials (like graphene, lipids, peptides, polymers, gold, and silver) are used.
The genetic material/drug is encapsulated into the nanoparticle or conjugated via electrostatic binding between the nanoparticle’s surface and genetic material/drug. For example, drug delivery with electrostatically assembled nanoparticles, researchers can do it with or without the help of polymers. So in general, researchers can either mix the drug with a polymer of opposite charge and thus formatting nanoparticles, or they can modify the surface of pre-formed nanoparticles with a negative-charge group and then positively charge the drug.
Their main advantages are good uptake and internalization and lower reticuloendothelial clearance. Some nanoparticles have cell-specificity.
Besides these, nanoparticles can be used beyond treatment for imaging and diagnosis.
Their disadvantages are lower transfection efficiency compared to viral vectors, potential toxicity and inflammatory response, and biodegradability.
Polymers can be natural (cellulose, chitin, starch) or synthetic (PLGA, PLA, PGA), and they are used for nanoparticle generation/ production.
They have low immunogenicity and are versatile and biocompatible. However, their production could sometimes be complicated and expensive (especially for synthetic polymers).
Analysis and findings of gene delivery systems used in clinical trials.
Procedure
In this article, we analyzed gene delivery systems employed in clinical trials we derived from Clinicaltrials.gov. Specifically:
- We searched for trials (regardless of their status) that included the phrases “gene therapy”, “gene transfer systems” and “gene delivery systems” and ended up with 767 unique NCT trials.
- We then filtered them by intervention (viral vs non-viral gene transfer system) and by major therapeutic areas /conditions.
Results
After having analyzed 767 clinical trials by status, by condition, and by intervention type and Gene delivery systems per therapeutic area along with their evolution through the years, we came up with the findings we visualize in the graphs below.
Clinical trials analysis by status, by condition, and by intervention type
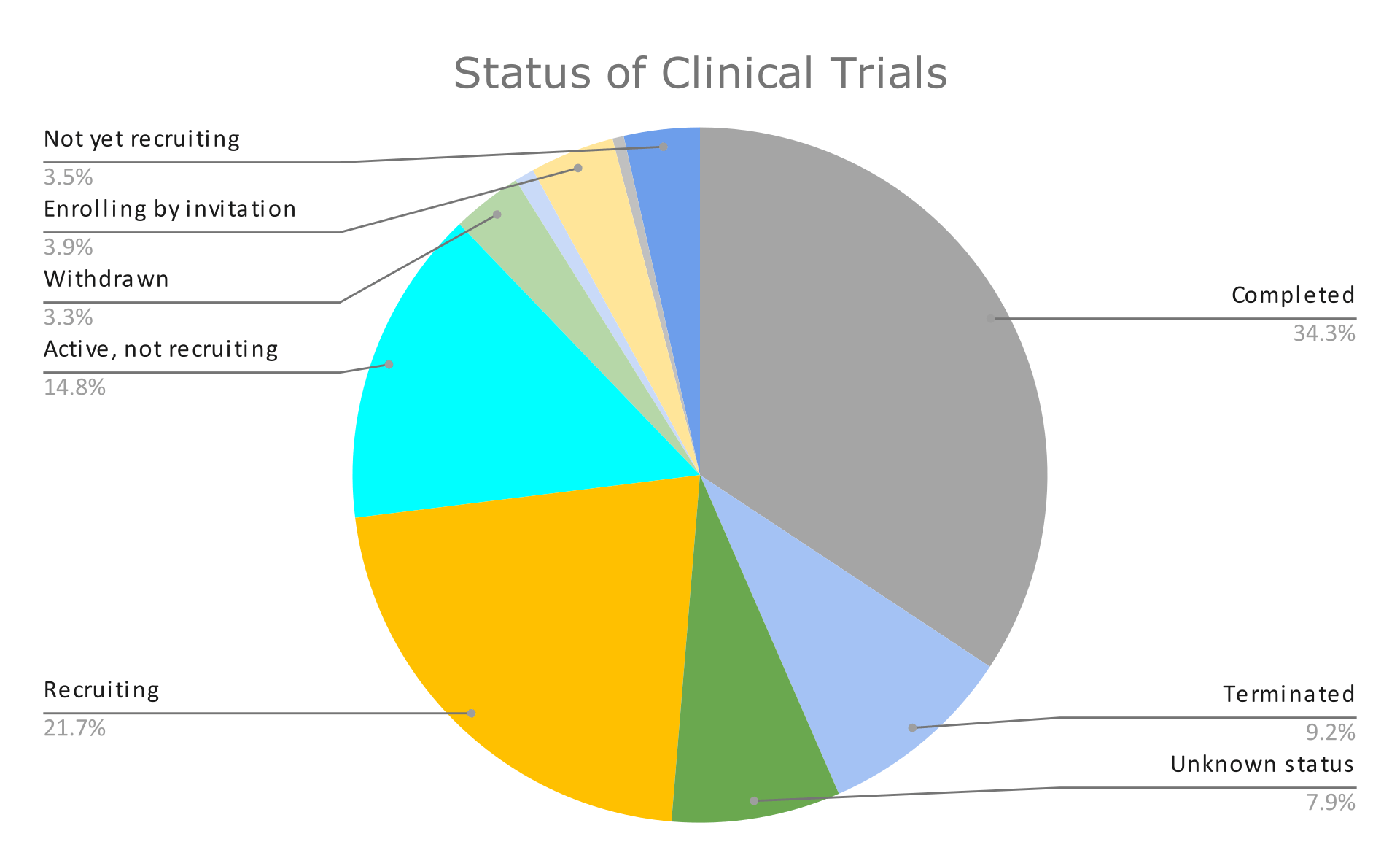
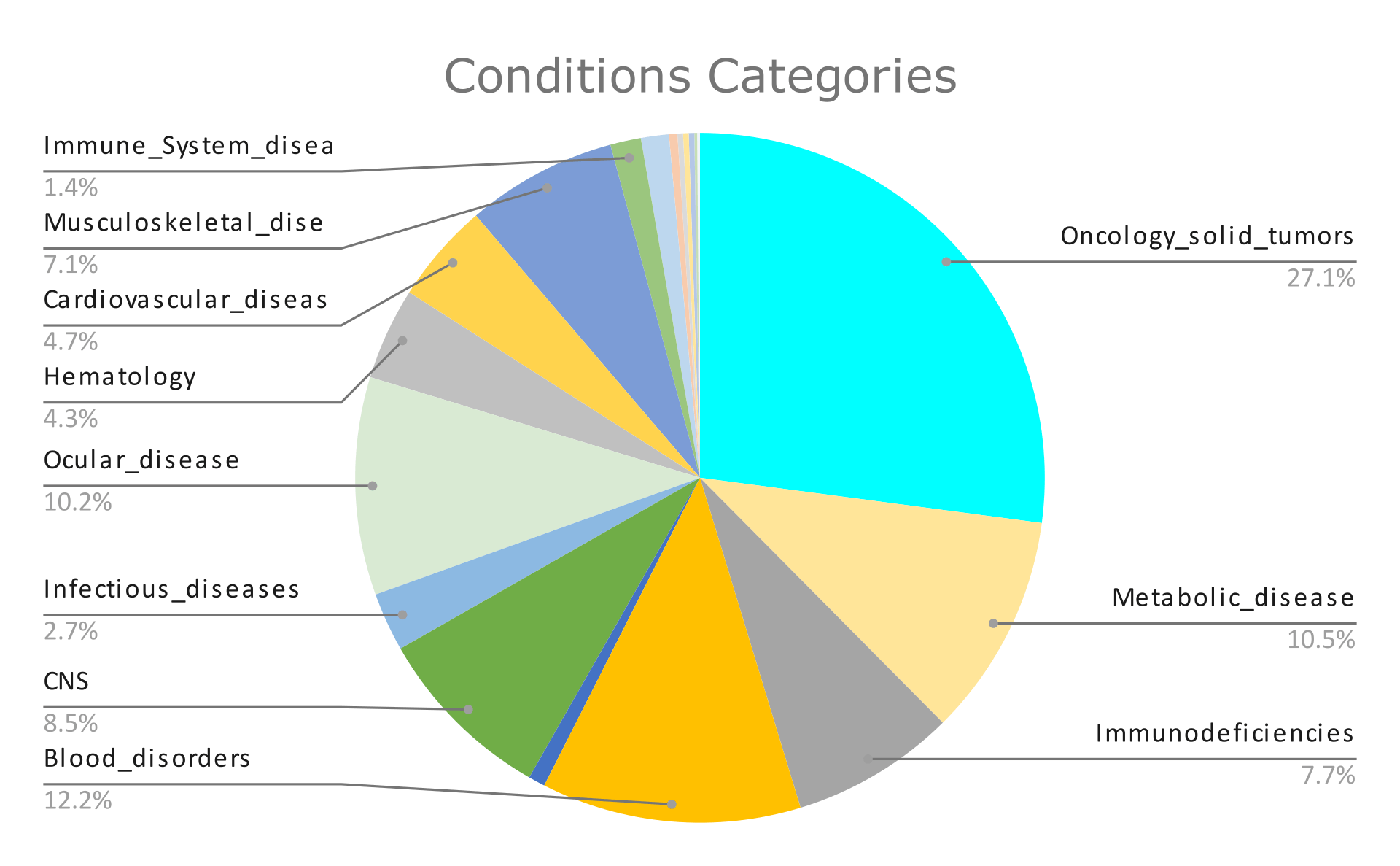
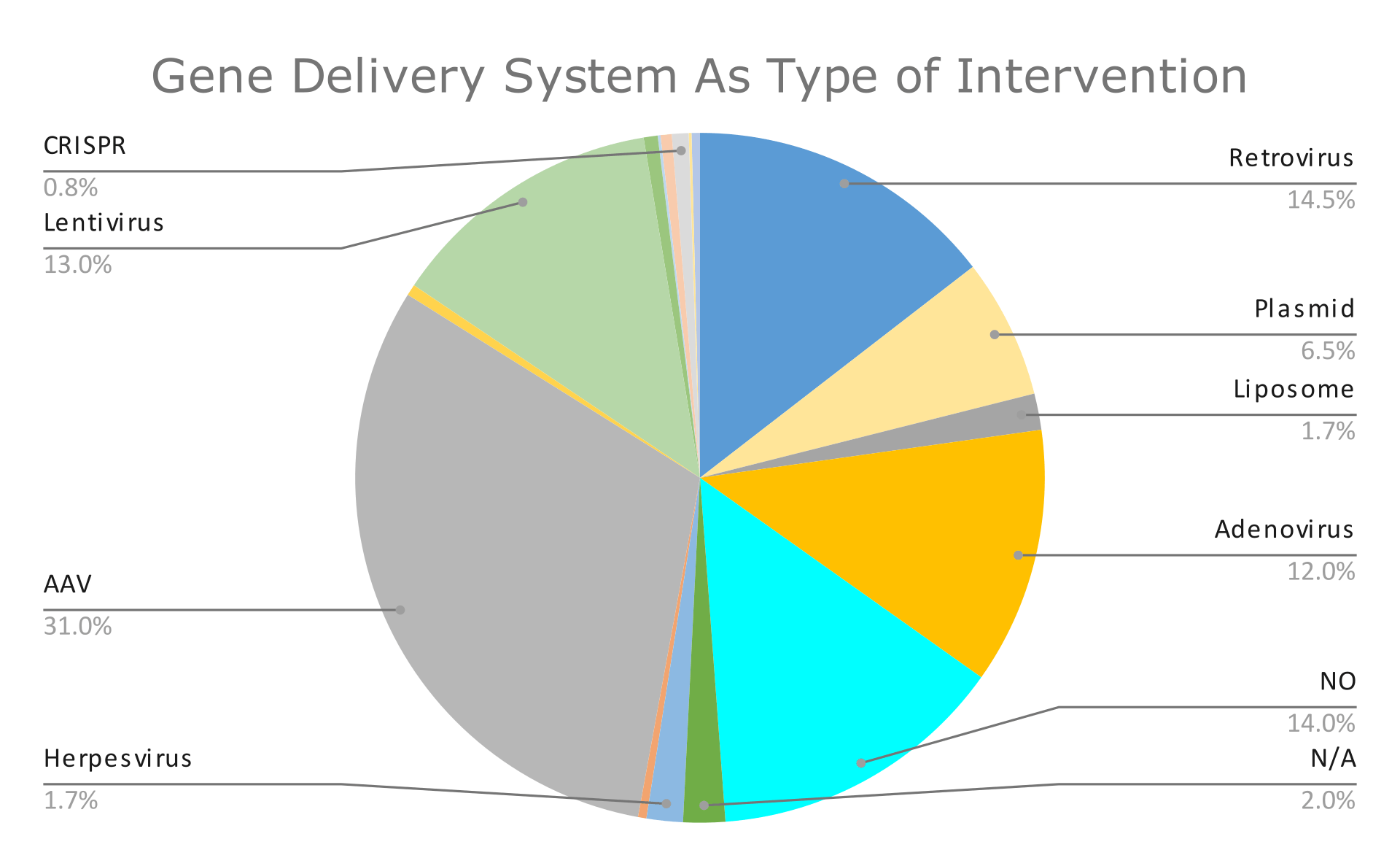
The graphs below summarize the findings of these 767 clinical trials. Graph 1 presents the percentage of clinical trials’ status, and Graphs 2 and 3 present the results we got after filtering by condition and intervention type, respectively.
Gene delivery systems analysis per therapeutic area
The graph below shows the number of clinical trials employing each one the Different Delivery Systems per therapeutic area.
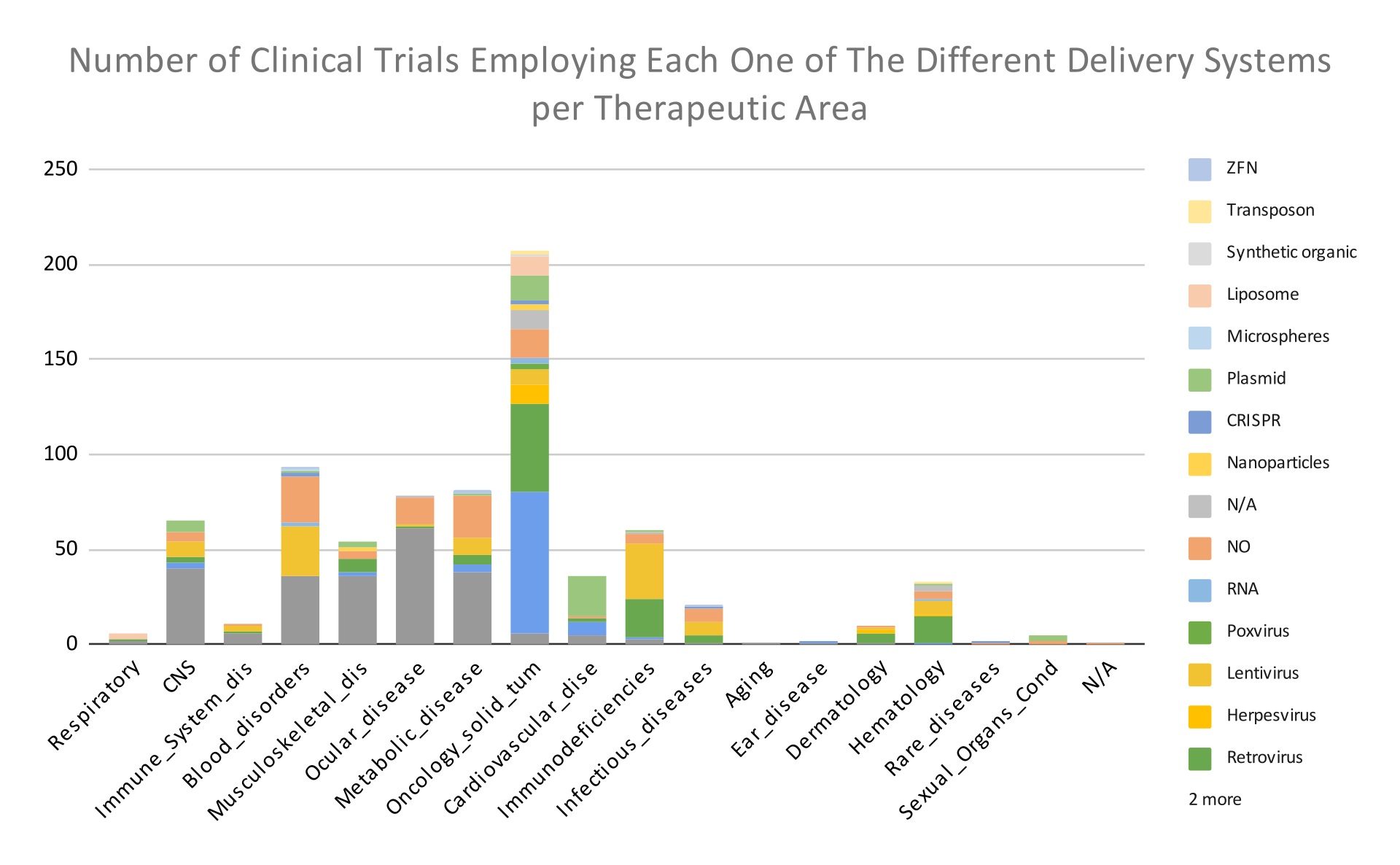
In graph 4, we observe that the most common gene delivery systems used in oncology are Adenovirus and Retrovirus; in blood disorders, AAV and Lentivirus; while in ocular, metabolic, and musculoskeletal diseases, AAV.
In graph 5 we see the number of clinical trials per condition that were conducted using each one of the two main categories of gene delivery systems (viral and non-viral). As mentioned above, the term NO stands for no intervention, while the term N/A stands for unknown intervention type.
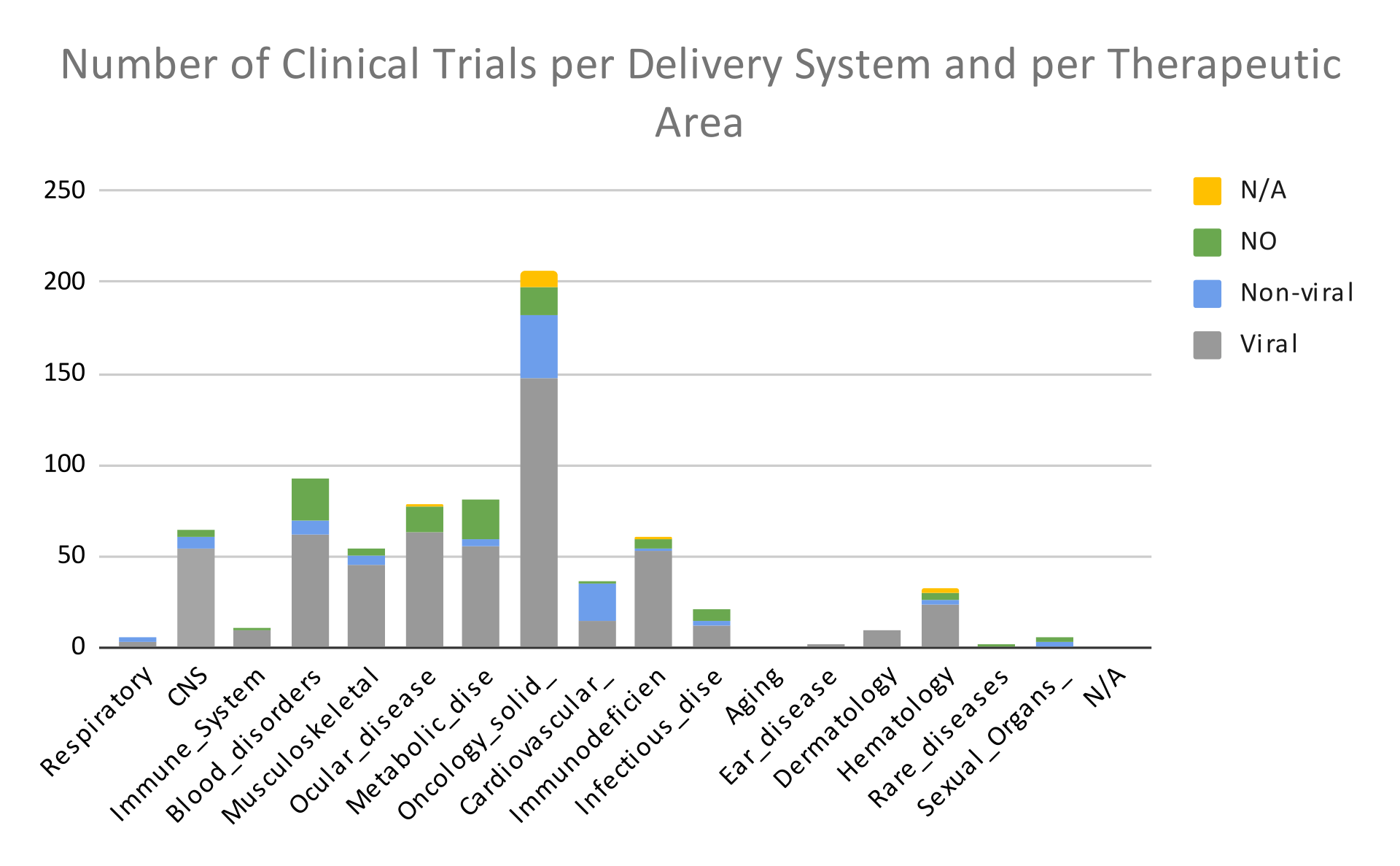
In this graph (5), we observe that the most common way to insert the transgene into cells is through the use of viruses. Below, we will discuss the reasons why.
Evolution of gene delivery systems through the years
Below, in graphs 6 and 7, we see the number of clinical trials and the type of intervention used in these trials from 1988 to 2023. We observe that through the years, gene therapy mainly employs viral transfer systems over non-viral ones. At the same time, we also observe that the number of trials using non-viral systems has increased over the last few years; below, we discuss this further.
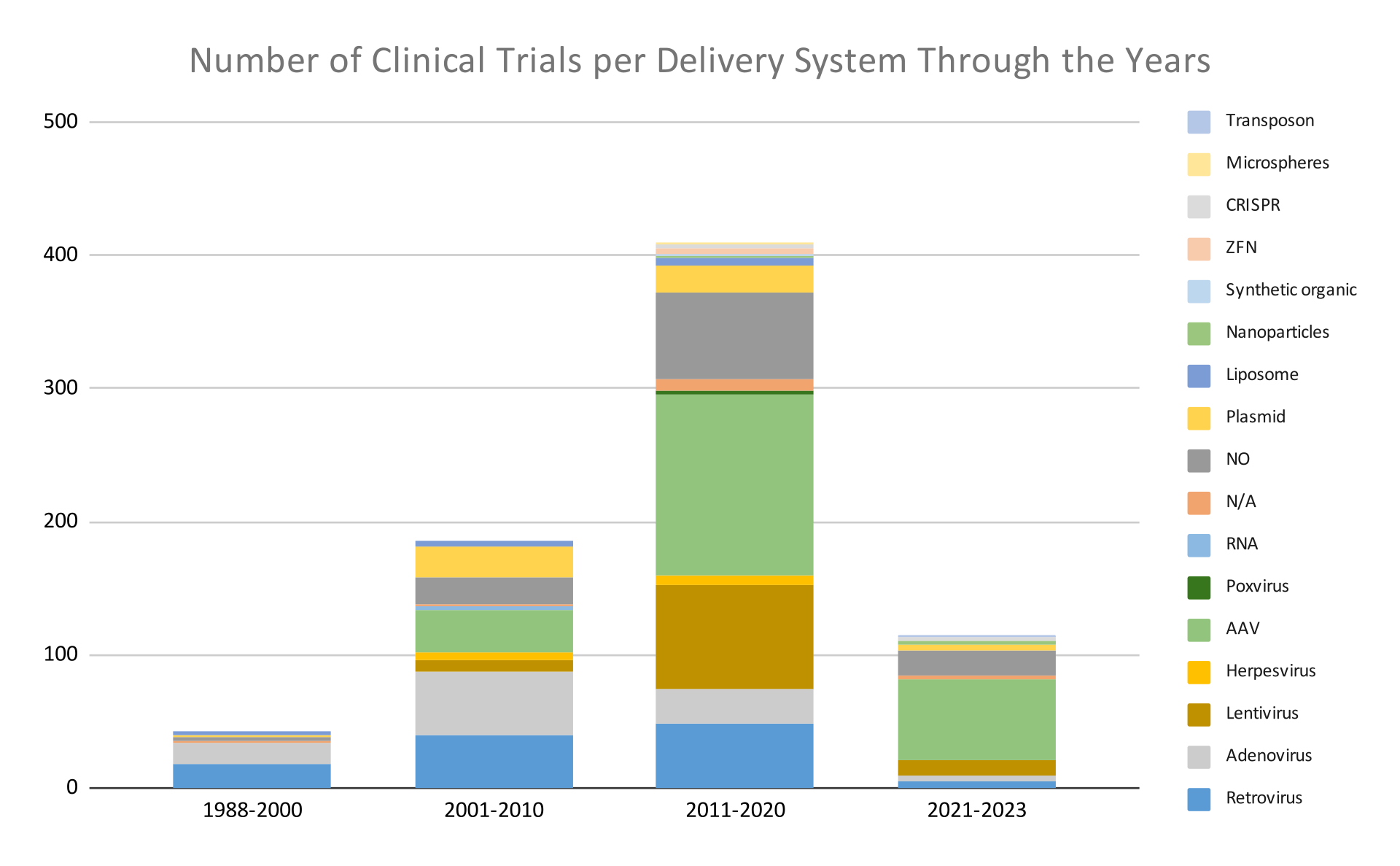
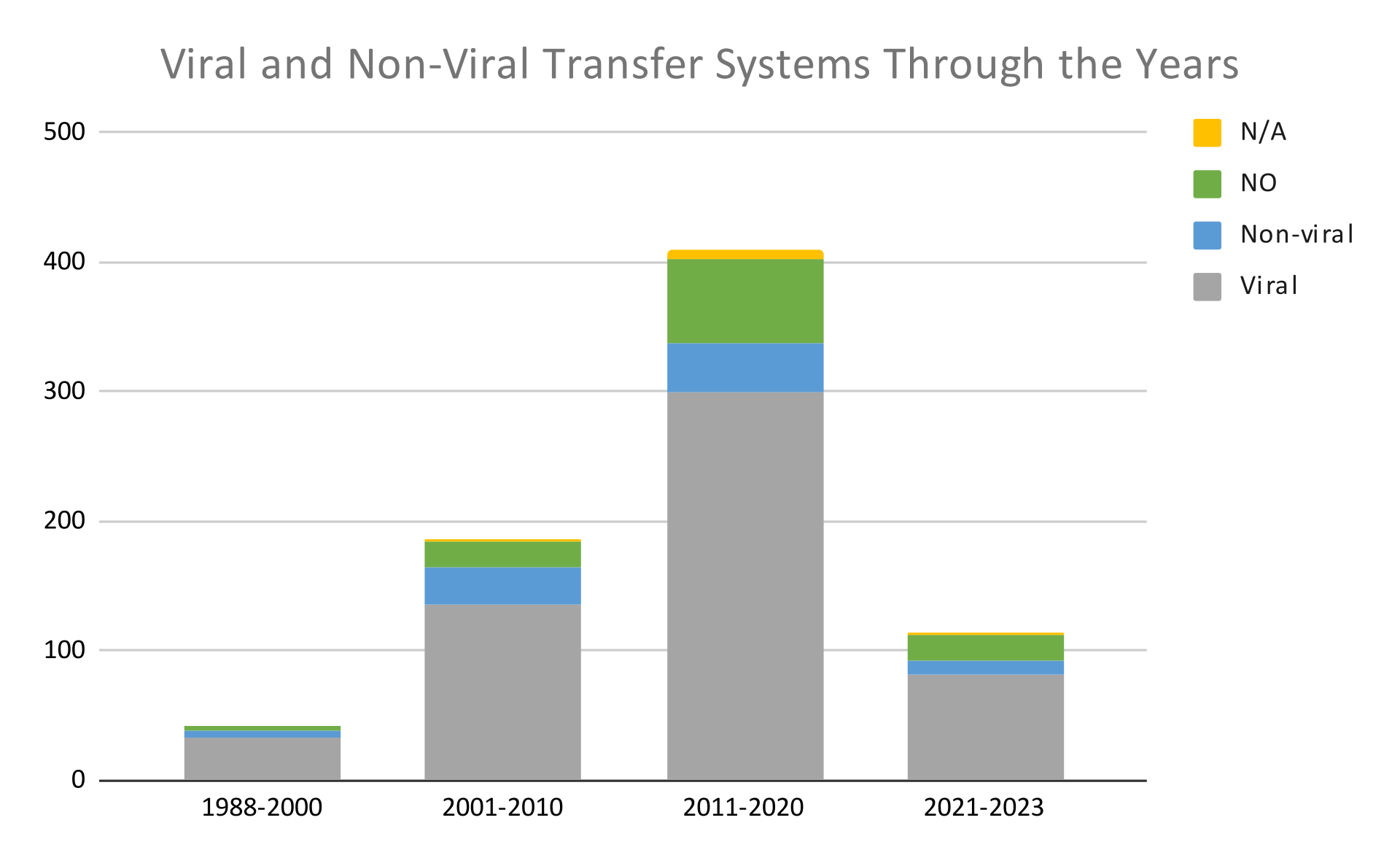
Research findings in a nutshell
From the above analysis, we deduced that up to the year 2020, viral-based gene delivery systems were used more commonly than non-viral ones. The extensive use of viral-based gene delivery systems can be explained by considering the advantages and disadvantages of each category, as summarized in the table below.
| Type of vector | Advantages | Disadvantages |
| Viral vectors | High transduction efficiency
Choice upon long-term or transient transgene expression Ability to target cells in G0 phase Cell specificity by using alternative envelopes |
Low virus titer
Difficulties in large scale production High cost Immune reaction to virus Potential toxicity, insertional mutagenesis and position effects Limitations of packaging capacity |
| Non-viral vectors | Simple manufacturing
Lower cost High packaging capacity Low immunogenicity |
Low transfection efficiency
Lower gene expression Potential toxicity related to materials used |
Gene Transfer Systems were in the spotlight during the 2023 J.P. Morgan Healthcare Conference.
During the 41st JPM 2023 Healthcare Conference, the viral gene therapy market’s latest updates were in the spotlight. The market is still evolving, and there is ongoing progress in research, innovation, and collaborations.
- Sarepta Therapeutics expects to generate $4 billion from US sales of the AAV-based gene therapy product SRP-9001 (if approved) for the treatment of Duchenne muscular dystrophy.
- BioMarin AAV-based Roctavian for hemophilia A is highly expected.
- BluebirdBio lenti-based lovo-cel for sickle cell disease is also highly expected.
- Spark Therapeutics announced positive interim results from its phase 3 trial of SPK-8011 for hemophilia A gene therapy (TA Hematology).
- Editas Medicine revealed new preclinical data on EDIT-301, a CRISPR-based gene editing therapy for sickle cell disease and beta-thalassemia. (TA Hematology)
- Fujifilm launched the BalanCD HEK293 medium to boost AAV production.
- 4D Molecular Therapeutics: Clinical trials for AAV-based gene therapy product, 4D-150, for therapy of diabetic macular edema and wet-age related macular degeneration.
- Neurocrine Biosciences joined forces with Voyager Therapeutics to develop Voyager’s GBA1 gene therapy program and three more gene therapy programs for neurological diseases based on Voyager’s next-generation TRACER capsids.
- Asklepios BioPharmaceutical and ReCode Therapeutics will combine CRISPR technology with lipid nanoparticle delivery technology to develop precision medicine drugs for liver and lung diseases. (non-viral market)
What we learned about gene delivery systems used in clinical trials and what lies ahead. Trends, threats, and expectations.
After giving an overview of viral and non-viral vectors and their characteristics, we conducted a (non-exhaustive) analysis of data from clinical trials related to gene therapy, gene delivery systems, and gene transfer systems. We observed that 1) gene delivery systems were mostly applied in oncology and in blood disorders and 2) through the years, viral vectors were used more than non-viral, with the most frequently used system being AAVs.
The market of viral and non-viral vectors is still evolving/expanding with several innovations, as highlighted in the JPM Conference 2023.
While viral vector systems accounted for the biggest share of the gene delivery market, this will probably change, and the non-viral market will have substantial growth.
The viral vectors’ high manufacturing cost and their increased immunogenicity compared to non-viral vectors have begun to alter the balance. The progress and innovation in non-viral systems, including the construction of biodegradable polymers, the promising use of non-viral vectors to regeneration medicine and tissue repair, non-viral based methods for CAR T cells generation, the rise of lipid nanoparticles and their use in mRNA vaccines are all contributing factors to the growth of the non-viral systems. Additional updates in the field of non-viral vectors include the approval of liposomal drugs Doxil and Onpattro.
As the field of non-viral vectors is rapidly evolving, we anticipate changes in the market. The future will show if and to which extent viral gene therapy might be under threat.
Do you want to stay updated with the latest updates in Gene Therapy?
Or do you need a partner to support you with competitive intelligence through the new drug development process?
Contact us and gain best-in-class market insights. Learn how we help you make informed decisions.
#genedelivery #genetherapy #viralvectormarket #nonviralvectormarket #clinicaltrials #vectorsmanufacturing #JPMconference2023
Sources:
Viral Vectors for Gene Therapy: Current State and Clinical Perspectives
Appraisal for the Potential of Viral and Nonviral Vectors in Gene Therapy: A Review
Nanotechnology in drug and gene delivery | SpringerLink
Gene Delivery: Applications and Future Potential | BMG LABTECH
Recent advances in the development of gene delivery systems | Biomaterials Research
Biodegradable Polymers for Gene-Delivery Applications | IJN
Global Viral and Non-Viral Vector Manufacturing Market.
Growth in Cell and Gene Therapy Market
Gene Delivery Technologies Market Size Report, 2021-2028
Lipid Nanoparticles: From Little League to the Majors
JPM23, Day 1: Pfizer CEO shares pandemic predictions; BMS touts new launches
JPM23: Partnerships Take off From JP Morgan Healthcare Conference 2023 Day One – GeneOnline News
Image Credits: