When thinking of health apps, the most common ones coming into our minds are digital platforms for appointments with doctors and smartwatches tracking physical exercise or monitoring medication.
However, the pandemic paved the way for a digital revolution at all levels because it raised the necessity for access to patient data or patient-physician communication without going to the hospital.
Now, under the digital umbrella, we encounter digital platforms facilitating communication (telehealth) and cloud services providing safe storage and quick access to patient documentation along with digital pills and algorithms making diagnoses.
Novartis Argentina and Clonify partnered to address access to treatment hurdles.
At this point, it is worth shedding light on how the Argentinean innovation ecosystem delivered a user-friendly platform for patients with asthma, lung cancer, and other diseases that facilitate access to medication by diminishing access to treatment time by 35%.
Novartis Argentina, aware of the patients’ challenges while navigating the healthcare system, collaborated with Clonify—an Argentinean startup participating in the “Startup With IBΜ” program and using the IBM public cloud—to develop the platform for patients.
Combining traditional treatment methods with digital tools improves patients’ QoL. Patients connect easily with their doctors and show improved adherence to their treatment.
Digital pill Abilify MyCite failed to keep its promise.
However, not all concepts lead to successful products, no matter how revolutionary they might be. As we discussed in our previously published article “Digital Pills: Hard to swallow offerings or absolutely necessary?” the much anticipated digital pill Abilify MyCite for monitoring psychiatric patients (diagnosed with bipolar disorder, schizophrenia, and other conditions) adherence has not taken off. Though the FDA approved the digital pill, Proteus (the company owning the IEM patent) went bankrupt. As FDA had described in 2017, no clinical benefit or increased compliance was shown due to its use.
Despite the initial enthusiasm, privacy and logistics issues did not favor the marketing of digital pills. Patients with paranoia as their main pathology will probably not welcome being monitored and controlled. And there are concerns about third parties’ (recruiters and insurance companies) access to personal data. After all, ethical, philosophical, and practical questions restrained their adoption.
To approve or not to approve? FDA on fostering digital health innovation responsibly.
Of the plethora of available health apps (>350,000), only a small fraction follow guidelines. For example, only a few hundred AI tools have been qualified by the FDA.
In 2020, the Medicines and Healthcare Regulatory Agency (MHRA) updated the App Guidance for compliance re-assessment of digital products and classification under medical devices regulations. This update imposed further regulatory requirements for digital apps and made recommendations on the clinical trials’ design. New recommendations encourage the employment of prospective studies, including concurrent data evaluation and device deployment (vs. retrospective, which are the most common till now in this space) and multi-site clinical trials to establish safety and efficacy robustness. (Figure 1, adapted from Nature_2021).
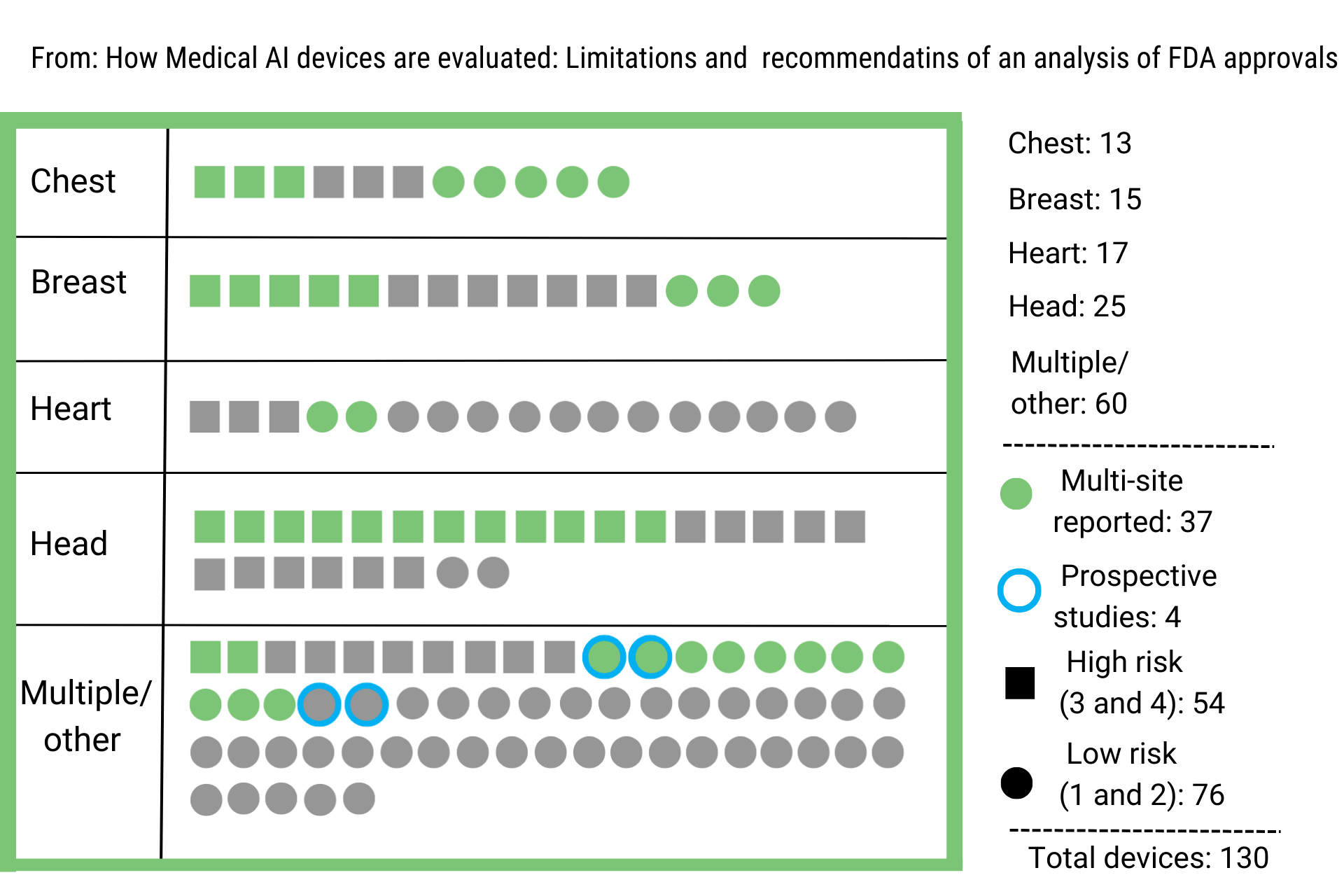
What type of digital health apps won the FDA race?
By now, most approved health apps either extract cardiac or neural signals or provide imaging methods (CT, MRI, X-ray) assistance. AI, ML, neural networks, and deep learning algorithms train a model and learn to recognize disease patterns offering HCPs valuable assistance. However, as the FDA takes the digital leap, new issues arise.
Do algorithms always get it right?
Concerns are raised about the nature of the algorithms and data used, the so-called “garbage in, garbage out” in informatics. Testing showing superiority or equivalence towards other predictive methods or traditional diagnoses would be strong evidence for an algorithm’s validity and efficacy. However, the 5 Vs— volume, variety, velocity, veracity, and value— of real data limit the algorithms’ applications and restrict their training capabilities.
The paradox of choice around digital therapeutics.
Too many options sometimes come with difficulty in making a good decision. So, another concern is which digital tools we will finally adopt in practice. It is rational to say that we will prefer those tools assisting HCPs without replacing them. However, we are curious to see if and to what extent this will be the case.
This is how personalized medicine will transform healthcare.
More is yet to come in the digital space, even concepts that till recently seemed imaginary. Personalized medicine is one of them. The vision of precision medicine is alive and kicking, with digital gene signatures envisioned to stratify patients into subgroups in the clinical landscape, providing a valuable tool in the hands of clinicians; patients’ molecular profiles will indicate which medication is more efficient.
The e-NIOS case. Embedding biological knowledge into AI.
The Greek spin-off, e-NIOS, was recently awarded the first prize by Pfizer Greece “Start4Health” acceleration program for developing an AI-centric omic platform.
Αdressing digital health challenges and unlocking a world of capabilities. Food for thought.
The digital health space capabilities are fascinating and digital health will be an inseparable partner in clinical practice, prognosis, and diagnosis.
However, the promising developments in this irreversible big data generation race go hand in hand with legitimate concerns and questions.
How are the regulatory mechanisms going to adapt? And how effectively will stakeholders handle the information to prevent sensitive data revealing? Ethical and practical issues have yet to be resolved, and mitigating regulations are to be fully formed.
Are you interested in the latest update in digital health applications and HealthTech?
Or do you need a partner to help you identify digital health investment opportunities?
Contact us and gain best-in-class market insights. Learn how we help you make informed decisions.
#digitalhealth #digitalmedicine #healthtech #FDAapproval #personalizedmedice #AIhealthcare #bioinformatics #digitalpill #clinicaltrials #trialdesign
Photo Credits: Designed by Freepik
Sources:
https://www.ibm.com/topics/artificial-intelligence-medicine
https://dataconomy.com/2022/11/big-data-and-artificial-intelligence/<a