The overuse and misuse of antimicrobials in humans and animals have escalated antimicrobial resistance (AMR). With almost 5 million deaths per year associated with bacterial AMR, of which 1.3 million deaths have bacterial AMR as the direct cause and projections stating that the latter number could reach up to 10 million by 2050, it comes as no surprise that WHO lists AMR as one of the top global public health threats today.
Lower antibiotic efficacy could turn ‘routine’ procedures into riskier ones because of infection. Especially people with an already weakened immune system, such as cancer patients undergoing chemotherapy or transplant patients, will be more likely to succumb to diseases.
The economics of drug discovery work against antibiotic development.
Research into new antibiotics suffers from decades of under-investment from both governments and companies. The easy targets for research have all gone and the lack of investments means that only very few new antibiotics have been released over the past two decades. The research and development costs associated with new antibiotics discovery, clinical trials, etc., along with scientific and regulatory barriers, are considered too much to be worth the rewards.
A new antibiotic compound targeting Acinetobacter baumannii brings hope for AMR.
Recently, scientists focused on finding a new way to treat infections caused by carbapenem-resistant Acinetobacter baumannii (Crab ) and have discovered an entirely new class of antibiotics. The above pathogen is classified as a priority one by the World Health Organization because it is resistant to many antibiotics currently available in the HCP arsenal, posing risks for invasive blood and chest infections.
This new drug, Zosurabalpin (Abx MCP, RG6006), is a small molecule novel chemical class of antibiotic and is being developed by Roche, more specifically by their Pharma Research and Early Development (pRED) group. They currently have two novel class antibiotics in development. Both are for gram-negative bacterial infections, one for the above-mentioned Acinetobacter baumannii and the other is focused on the treatment of carbapenem-resistant infections. Michael Lobritz, Head of Infectious Diseases at pRED said: “Importantly, regulatory guidelines facilitate this, allowing us to use highly translatable animal model data to complement leaner clinical trials in support of registration.”
Using structural, biochemical and genetic approaches, the team identified a new class of antibiotics that target the outer membrane integrity of the bacterium Acinetobacter; this membrane is excellent at protecting the bacteria from outside threats. Their findings may have identified a new way of treating other Gram-negative pathogens.
AI-guided discovery against Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci.
Meanwhile, another team of researchers has employed deep-learning and large chemical libraries to find a structural class of antibiotics, one of which is selective against methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci.
Push and pull strategies for shifting the focus of pharma to antibiotics.
Even though the discoveries mentioned above sound promising, there is still a mountain to climb to tackle the ARM problem.
An article published in the journal Infection and Drug Resistance identified numerous types of push and pull incentives that could be put to good use by governments around the globe to encourage the development of new antibiotics.
Push incentives include grants, subsidies, and tax incentives. They have advantages, such as lowering the cost of R&D and funding projects that SMEs may lack the capital to pursue. Still, they also have disadvantages, as there is the risk of funding a project that will fail, or there might be a lack of applicants due to a perceived belief that they will not receive funding.
Pull incentives include advance market commitments, exclusivity patent extensions, market entry rewards, and tradeable vouchers. They, too, have advantages and disadvantages; the advantages include that they will reward successful antibiotics and lower manufacturing risk. There is also the disadvantage that the incentive to maximize sales remains and that resistance to the antibiotic might occur during patent extension periods.
Increased investment, robust policies, and international collaboration are keys to the AMR crisis resolution.
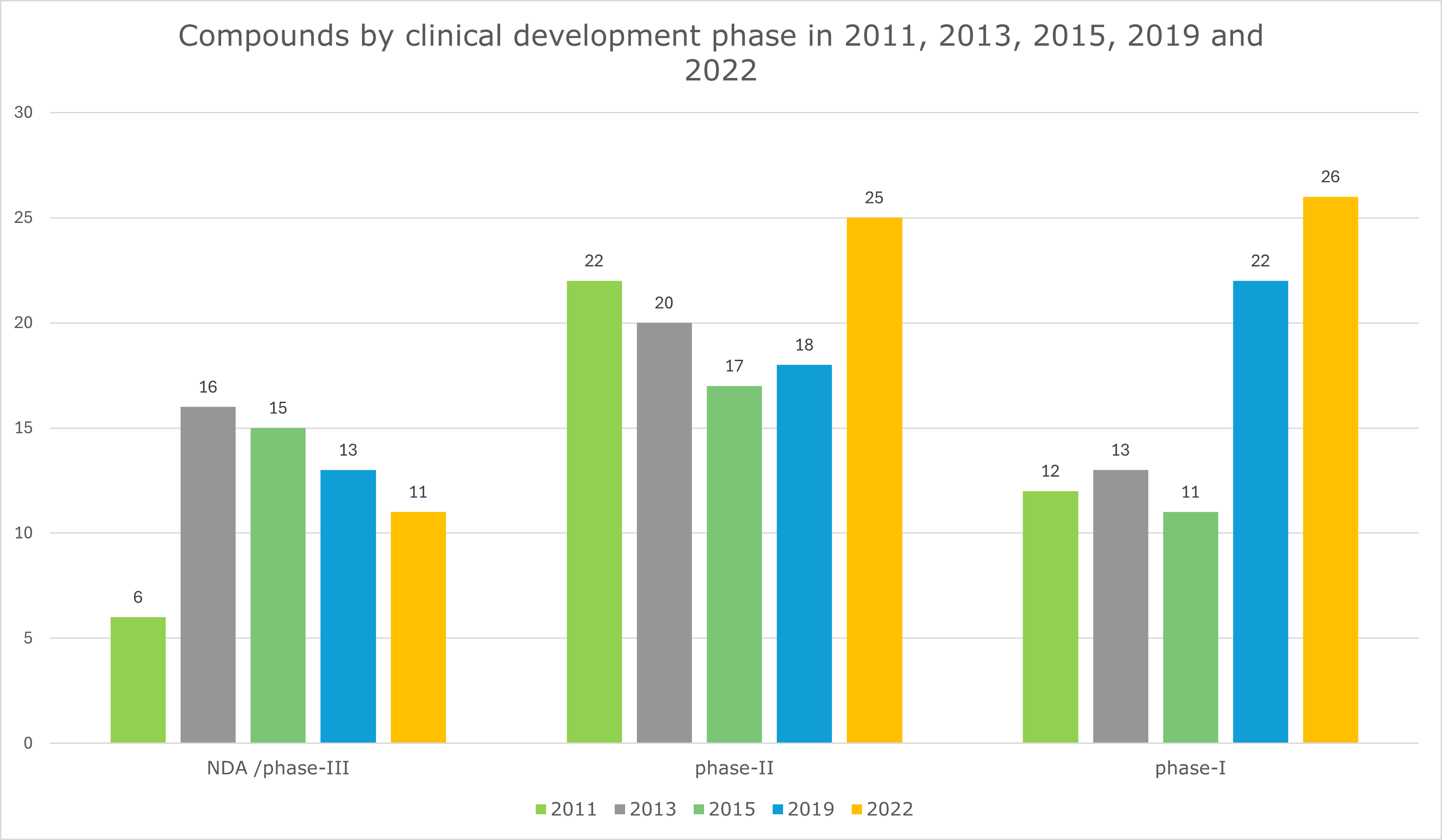
With only 62 new antibiotics (Graph 1) in the development pipeline in the last decade, more investment by healthcare systems in approaches to reduce antimicrobial resistance (AMR) and introducing new policies are vital parts of the solution, but they are not the only answers.
Graph 1: Compounds by clinical development phase in 2011, 2013, 2015, 2019 and 2022. Data taken from the article “Antibiotics in the clinical pipeline as of December 2022” ( by Butler, M.S., Henderson, I.R., Capon, R.J. et al.) published in The Journal of Antibiotics 76, 431–473 (2023).
Effective management of AMR requires a multifaceted approach that involves various stakeholders, such as governments, pharmaceutical companies, various regulatory agencies such as FAO (the Food and Agriculture Organization of the United Nations), NGOs, and not-for-profit organizations such as GARDP (Global Antibiotic Research & Development Partnership).
Meanwhile, researchers should start considering non-antibiotic solutions to fight bacterial infections. Cost-effective local strategies, such as educational programs focusing on optimized antibiotic usage targeting HCPs and public awareness campaigns, could impact demand until a breakthrough solution is available.
Do you want to stay updated with the latest advancements in addressing AMR and other today’s healthcare challenges?
Contact us and gain best-in-class market insights. Learn how we help you make better informed decisions.
#FightAMR#AMR #AMRcrisis #antimicrobioticspipeline #drugresistance #antimicrobialresistance #GARDP#AntimicrobialResistance #StopSuperbugs #InfectionControl #GlobalHealth #PublicHealth #HealthcareSolutions #Acinetobacterbaumannii #MRSA #MedEd #AMRawareness
Sources:
https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)02724-0/fulltext#%20
https://www.ers.usda.gov/webdocs/publications/45485/err-200.pdf
https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf
https://www.nature.com/articles/s41586-023-06799-7
https://www.bbc.com/news/health-67881289#comments
Discovery of a structural class of antibiotics with explainable deep learning | Nature
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7872909/
https://gardp.org/about-gardp/
https://www.roche.com/solutions/pipeline
https://www.roche.com/solutions/pipeline
https://www.roche.com/stories/new-era-of-antibiotics